Double Fruited with Two Seasons of Peaches
Some recipes I brew are inspired by science and some beers inspire me to dig further into the science after they are brewed; this particular beer did both. The science of the recipe was inspired by research where early fruiting (like early dry hopping) might push bioflavoring between fruit and brettanomyces. The fun part of the plan was to double fruit the beer, where the first peach addition would be added during active fermentation from the 2016 harvest. The second fruit addition would come after aging and acidifying and from an entirely new season of 2017 peaches from the same farmers market and potentially the same grower. Tasting the beer is what led me to dig into the research on quinoa in general, peach disease control, and a new role for alpha acids to play from dry hopping. After talking with some experts in their respected fields and multiple lab tests, this beer taught me a lot.
Brewing with Quinoa
About a year and a half ago I chose to use flaked quinoa as a large percentage of the grist in this beer, and I have no memory or notes as to why. More than likely I thought the high protein content in quinoa might help with the mouthfeel. Not knowing exactly how quinoa might behave, I decided to give the literature a read to see what researchers have discovered when brewing with quinoa.
If any flavor comes through when using flaked quinoa, it’s likely to be slightly nutty and earthy. I follow the general directions when making quinoa to eat, which is to rinse it to avoid a “dirt-like” taste, which is probably a good idea when brewing with it as well. Quinoa has a peak gelatinization onset, peak and end temperatures of 125 °F, 136°F, and 147°F1, which suggest you could just toss raw quinoa into your mash when used as a portion of the grist after rinsing. If using high percentages of raw quinoa, external enzyme supplementation would be required, however, you can always opt for the flaked version, which I did in this beer.
Quinoa can be malted, and some of the studies have done this, however the small grain size makes the processes difficult. The fermentable makeup of quinoa is also different than typical barley malt as quinoa’s fermentables have a higher percentage of glucose. A study coming out of Belgium looked at brewing beer by replacing pale malted barley with either 20% or 40% raw (unmalted) Belgian and Peruvian Quinoa and tested and compared the results to a 100% pure malt beer in small 1L batches. For the most part, the wort parameters were reasonably similar between the quinoa beers and 100% malt beer. The quinoa produced a similar color beer, had a slightly higher pH, similar viscosities, lower FAN, lower fermentable sugars, and a higher percentage of protein. Specific to protein, when 20-40% of the grist was replaced with quinoa, the protein percentage of the wort increased by about 2%.2
One area the raw quinoa beers differentiated from the 100% malt beer in the study mentioned above is the higher total polyphenol content coming from the quinoa. The quinoa beers averaged 234.5 mg/L of polyphenols. Unfortunately, the paper didn’t list the 100% pale malt polyphenol content. I was able to find a study that measured the polyphenols of a 100% malt beer (no hops), and the beer had 137 mg/L of polyphenols. So, when compared to all-malt, substituting just 20-40% of the grist with raw quinoa could come close to doubling the polyphenol. The polyphenol content is something to consider if making an extremely hoppy beer, where even more hop polyphenols will be added, which I think can lead to an astringent vegetal bite.
Recipe
| Original Gravity | Final Gravity | Final pH | Water | Mash Temp. |
|---|---|---|---|---|
| 1.054 | 1.002 | 3.4 | R/O | 1 gram/gal Calcium Chloride & 0.5 gram/gal Gypsum | 152°F |
| Grain | Percentage |
|---|---|
| Briess White Wheat Malt | 48% |
| Flaked Quinoa | 26% |
| Malted Red Wheat | 18% |
| Carapils | 7% |
| Acidulated Malt | 1% |
| Hot-Side Hops | Amount | Addition |
|---|---|---|
| Columbus | 30 grams | @ 20 minutes (10 IBUs) |
| Yeast | Temperature | Duration |
|---|---|---|
| The Yeast Bay Amalgamation, and bottle dregs from Tired Hands Freedom From the Known | Ambient approx 68°F | Two Week Primary, 11-months in secondary, and 3-weeks on peaches. |
| Fruit | Amount | Time |
| Local Organic Peaches | 3 Pounds | Three Days into Primary Fermentation |
| Local Organic Peaches | 8 Pounds | Racked onto Peaches 11 months post brew day. |
| Dry Hop | Amount | Duration |
| Citra Lupulin | 28 grams | For Two-weeks @ room temperature after aging and fruit additions (more on why below). |
Results
The reason for the three pounds of local peaches during active fermentation was to encourage biotransformations with the Brettanomyces in the yeast blend used to promote a baseline fruit flavor. Bioflavoring between the peaches and Brettanomyces can happen by releasing glycosidically bound flavors in the peaches with an enzyme called beta-glucosidase that Brettanomyces can produce and is often not produced in high concentrations in Saccharomyces strains.
My tasting notes three months into aging noted that there was a metallic “penny-like” taste in the beer. I also pointed out that the beer was a bit sweeter than I typically notice at this point in the processes, which I hope I can attribute to the early fruit addition. After 11-months of aging, I went to the farmers market again to purchase the same local peaches I did the previous year and let them sit out for a about a week to get soft before adding them to the beer. I gently pulsed the 8 pounds of peaches in my Vitamix to try and encourage more extraction during the fruiting, but I regret doing this because the peaches wanted to float to the top and not stay in the beer. After three weeks on the peaches, I transferred the beer to a serving keg.
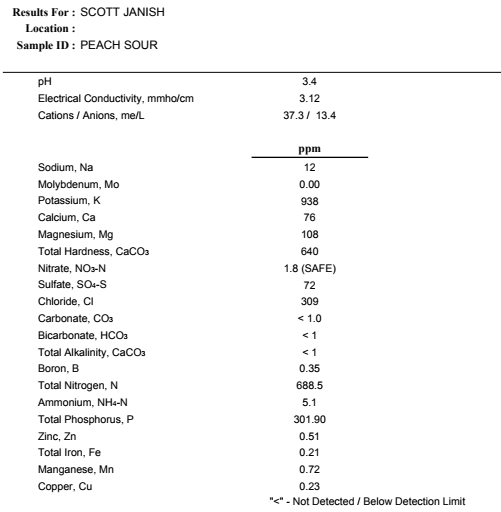
I had mix reactions when first tasting the beer, I could pick up on the peaches and I was happy with the acidity level, but that same metallic flavor and aroma I noted early on in the processes was still evident, but now even a bit stronger. Suspicious if my taste buds were on to something, I sent a sample of the beer to Ward Laboratories, Inc. to get tested for metals (test results below).
Does anything stand out to you in the results other than I misspelled quinoa on my submission sheet? For the love of Copper man, this beer was tested extremely high at 4.96 ppm! I think we found the reason for the metallic penny taste! Brewers want copper levels extremely low in finished beer to both to avoid a metallic taste as well as to prevent the quick oxidation of beer via the Fenton reaction. I recently had a hazy IPA tested that came back at only 0.26 ppm of copper, and the recommended level of copper in wort is <0.25 ppm3 This quinoa and peach beer is about twenty times the recommended limit of copper!
The World Health Organization recommends that adults should not exceed 10 mg/day of copper through water, food, or nutritional supplements,4 a value based on protection from liver damage as a critical adverse effect.5 so it looks like I can safely consume 4.5 pints of this quinoa peach beer before I’d reach the recommended daily limit of copper. Combining high levels of copper with alcohol doesn’t seem like a wise move for the liver, but I’m not a doctor.
Copper and Quinoa
Where is the copper coming from? Looking at a study of beers were made with either 100% malted quinoa or barley found that copper levels were substantially higher in the quinoa beers. The malted barley beer measured just 0.018 mg/L of copper in a fresh beer whereas the quinoa beer measured 0.075 mg/L, which is about four times the amount found in the barley beer.6 Because copper is known to absorb sulfur compounds, I wonder if a little quinoa in a lager could have an impact on reducing final beer sulfur concentrations?
Another potential copper factor to consider when brewing with quinoa is that repitching of yeast used to ferment high quinoa worts may lead to an even higher amount of copper in the beer. Generally, in 100% barley fermentations, the uptake of copper essentially eliminates it from wort, with quinoa, the higher levels present in the grain leaves more after fermentation. When repitching of quinoa worts was studied, with each successive fermentation, the uptake of metal ions (like copper) declined.7
So, it’s likely that some of the copper in this beer was from using a large percentage of flaked quinoa, however, based on the research, it seems very unlikely that all of the 4.96 ppm was quinoa’s fault. Where else could all the copper be coming from? It makes sense to next look at peaches, the other non-traditional ingredient in the beer.
Peaches and Copper
Fruit and vegetable plants can take up heavy metals by absorbing them from airborne deposits, through metal-rich soils via the root systems, and by the water used during throughout the plant’s life.8 So it does seem possible some pickup of copper could have happened, but enough to explain the massive amount in this beer? Knowing nothing about growing peaches, I started researching various treatments used to control diseases when I stumbled across an article titled, “Peach Disease – Bacterial Spot Differentiation from Copper Injury.” on the Penn State Extension.
The article was written by, Kari Peter, PH.D. and Assistant Professor of Tree Fruit Pathology who has expertise in various fruit diseases like peaches. Peter writes that copper will kill fungi and bacteria and when a copper solution is sprayed on peach trees, the copper ions are gradually released from copper deposits each time the plant surface becomes wet with rain. This gradual release of copper protects against plant pathogens and acts as a fumigant/bactericide treatment.
There is a high probability the copper came from the peaches.
I contacted Kari and explained that I was trying to figure out the source of the high copper content in a beer which had contact with 11 total pounds of peaches. I asked if it was at all plausible that the treatment of the trees with a copper solution for disease prevention could be a major source of the metal. Kari was kind enough to reply and said: “there is a high probability the copper came from the peaches.” However, Kari also said that the variety of the peach plays a role as well as the region it was grown in.
Kari went on to explain that bacterial spot is a troublesome disease for east coast peach growers and there aren’t very many control options, which is why copper is often used (it’s also the most effective for managing the disease). Both the peaches and I live on the east coast, so it seems we are onto something.
When treating the trees, there are several copper products that can be used up until the day of harvest; however, there are many where you have to stop using them 21 days before harvest. Another variable is the amount of rainfall during the peach growing season because the rain causes the copper to wash off. So, if the peach variety is very susceptible and disease conditions are favorable, growers will be treating their peaches every 7-10 days which means that peaches can have 10+ disease prevention sprays/season (doesn’t mean every treatment was copper-based however).
I figured since my peaches were organic, all of this might not even matter. I was wrong on this assumption though as it turns out that copper treatment is the natural option for a lot of diseases, so buying organic isn’t the answer to avoiding copper it seems. To avoid the issue, Kari recommends using peaches that are more resistant to bacterial spot, because of the genetics of such varieties, they require fewer copper treatments.
Michigan State University has an excellent guide as to what types of peaches are more prone to bacterial spot, which would be more likely treated with copper. It seems best to choose varieties for use in beer that are either in the excellent or good category for resistance to the disease.
Alpha Acids and Copper
At this point, I should probably stop thinking about this beer and start enjoying it, but there was research already on my mind for the advanced brewing book on hop-forward beers I have been writing. For the book I have done some research and writing about the ability of alpha-acids to complex metals like iron and copper. Because I was already in contact with Dr. Philip Wietstock from Technische Universität Berlin who had answered a few questions I had for the book on the subject, I also asked about this particular beer and potentially removing some of the copper via dry hopping. The idea is that dry hopping will introduce alpha-acids to the beer, which could potentially reduce the copper levels and maybe even the metallic aroma and taste. I was told that I should see a drop in copper if I decided to dry hop the beer, but that I shouldn’t do it considering how much time and effort had already gone into the beer. However, after telling me not to do it, Wietstock ended the exchange by saying curiosity-wide, “I’d do it.” Good enough for me, let’s experiment some more with this beer!
After a bit of discussion on the best way to do it, I first bottled of a handful of pre-dry hopped beers from the keg to compare to the dry hopped version later. There are a few things to consider when adding the dry hops for alpha-acids purposes at this point in the beers life. For one, the low pH of the beer (3.4) will affect the alpha-acid solubility as will the colder serving temperature, which could ultimately affect the amount of complex formation with copper. In an attempt to try and encourage alpha-acids solubility, I chose to bring the keg back to room temperature for an extended dry hop of two weeks. I first purged the hops as best as I could with CO2 and then added them to the keg in a fine mesh nylon bag and purged the keg a handful of times to try and minimize oxygen after the hops were dropped in.
I wanted to overdo to the alpha-acid content a bit for experimentation purposes, so I dry hopped with one ounce of Citra Cryo Hops® (AA% 24-26) to what remained of the keg, which I estimated to be about 2-gallons. Cryo is higher in alpha-acids (about double pellets), so it’s as if I dry hopped at about 1 ounce per gallon, not crazy, but higher than I probably would have done with a beer that was already fruited in two separate additions! I’m also hoping the solubility of alpha-acids may increase with the less vegetal material.
After dry hopping for two-weeks, I put the keg back in the fridge for another two-weeks (with the dry hops remaining in the keg) before pulling a sample of the beer and comparing it to a bottle I saved pre-dry hop. I had no problem identifying which beer was which by aroma alone, which isn’t unexpected after dry hopping with Citra. The Citra dry hopped beer had a softer aroma that was much more candy-like (think of a bag of Trolli Peachie-Os compared to fresh peaches). The pre-dry hopped beer had a sharper funky edge that still obviously had a metallic copper aroma but hiding underneath was still a bit of fruit.
My impression after tasting the the two beers was similar to the nose, the dry hopped beer was softer to me and less aggressive. Even the mouthfeel was a bit softer. The Citra dry hopped beer was fruitier, almost as if I artificially sweet in a way, with the finish tasting a bit like a sweet tart. Compared to the non-dry hopped beer the fruit flavor with Citra dominated and was lasting compared to being muddled and hidden behind the tartness and metallic funk in the non-dry hopped beer. Overall, both beers are good, but there is no question I prefer the dry hopped beer. Do I think the copper levels were lower based on aroma and taste? The dry hopping did seem to reduce the metallic aroma but perhaps Citra was just overpowering it. The only way to know for sure was to send another sample of the dry hopped version of the beer to lab for more testing (below).
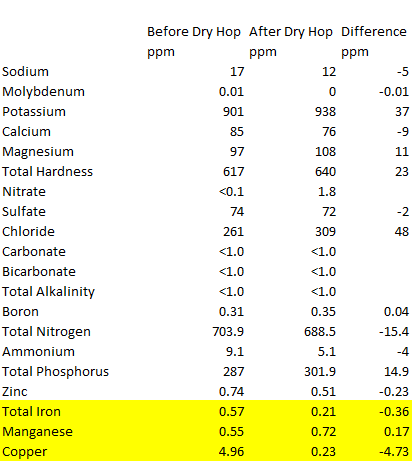
As you can see from the results, I avoided misspelling quinoa this time on my submission sheet by ignoring it all together. You can also see from the results that the copper levels were dramatically reduced, all the way to below the recommended <0.25 ppm level for beer! I expected to see a drop in copper, especially after tasting the beer, but I didn’t think it would be this massive. Going from the starting copper level of 4.96 ppm to 0.23 is about a 21-fold reduction.
Below is a side-by-side of the lab results before and after dry hopping with the column on the right showing the differences. Of note was that the level of iron also dropped by more than half the original level, another potential source of oxidation problems in beer. Just as interesting was that that manganese did not reduce with alpha-acids and it actually increased from the manganese concentration from the dry hops themselves. The increase of 48 ppm in chloride is also interesting and may play a small role in the slightly softer mouthfeel in the dry hopped beer, but I can’t explain the increase.
I’m guessing that alpha acids are not a factor most brewers consider when dry hopping and at only 10% as bitter as iso-alpha-acids, that’s probably with good reason. However, they will dissolve into beer during dry hopping and can have an impact on bitterness, especially in conjunction with humulinones. In fact, I had a beer tested using HPLC for alpha acids in a beer that was only dry hopped (no hot-side or whirlpool hops at all) and it had 71.5 ppm of alpha acids, which is equivalent to about 7 IBUs of iso-alpha-acids. Although the bitterness impact is relatively small, it’s interesting the other impacts alpha acids can have, like reducing problematic metals like iron and copper.
I didn’t know what I was getting myself into when I brewed this beer back in 2016. The plan was to brew a sour beer with the unique idea of using peaches from two different seasons from the same grower and test out some fruit and bioflavoring research. But it was fun letting the beer steer me around the literature, first learning about quinoa then peach disease control and then incorporating research on hop acids with some surprising results. Big thanks to both Kari Peter and Dr. Philip Wietstock for answering my questions and sharing their knowledge!
Footnotes
- Hager, A., Taylor, J., Waters, D., & Arendt, E. (2014). Gluten free beer- A review. Food Science & Technology , 44-54.
- Pietercelie, A., Lepoivre, C., & Van Nedervelde, L. (n.d.). Comparative brewing performances of quinoa, amaranth, einkorn, millet, and chia to produce beer. Department of Brewing Sciences and Fermentation Technology.
- http://www.craftbrewersconference.com/wp-content/uploads/2015_presentations/R0900_Ruth_Martin.pdf
- www.who.int/water_sanitation_health/dwq/chemicals/coppersum.pdf
- Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington (DC): National Academies Press (US); 2001. 7, Copper. Available from: https://www.ncbi.nlm.nih.gov/books/NBK222312/
- Deželak, M., Zarnkow, M., Becker, T., & Košir, I. J. (2014). Processing of bottom-fermented gluten-free beer-like beverages based on buckwheat and quinoa malt with chemical and sensory characterization. Journal of the Institute of Brewing. doi:10.1002/jib.166
- Deželak, M., Gebremariam, M. M., Zarnkow, M., Becker, T., & Košir, I. J. (2015). Part I: the influence of serial repitching ofSaccharomyces pastorianuson the uptake dynamics of metal ions and fermentable carbohydrates during the fermentation of barley and gluten-free buckwheat and quinoa wort. Journal of the Institute of Brewing, 121(3), 356-369. doi:10.1002/jib.244
- Elbagermi, M. A., et al. “Monitoring of Heavy Metal Content in Fruits and Vegetables Collected from Production and Market Sites in the Misurata Area of Libya.” ISRN Analytical Chemistry, vol. 2012, 2012, pp. 1–5., doi:10.5402/2012/827645.





-610x915.jpg)
Scott Janish is the prince that was promised..
Nice work.
Another great article/research! The time and energy you put in this is astonishing.
I’m a fan of yours and Michael work so if i manage to visit east coast from Europe, visiting your brewery will be on my list! 🙂
Thanks, I hope you can make it, would love to pour you a beer!
Thank you Scott for the in-depth research. You went above and beyond to close the loop on this one! I would not have guessed that dry-hopping would reduce the copper concentration. I wonder if the dry-hopping increased the pH and precipitated out the copper and iron. A 2014 paper by Schmick showed dry hopping increasing the pH. However, I saw in your results that the pH did not change between the samples. Perhaps, re-carbonating the dry hopped beer brought the pH back down to 3.4, but that was not enough to redissolve the copper and iron minerals (at least at the time of sampling). It will be interesting to see if the copper concentration/flavor creeps back in over time.
Good luck with the brewery! After having read about your beers, I’m excited to finally try them.
Awesome post and interesting results. On a similar note, have you ever gotten dill or pickle notes from dry hopped sour beers? I am really curious as to where it comes from. I’ve noticed it more than a few times
I have from a few hop varieties (mainly sorachi ace), but it’s not something I’ve noticed across all dry hops sours though.
Hi Scott,
So I’m a little confused here. You say that dry hopping the beer should reduce the amount of copper via chelation with the alpha-acids. I can agree that this would be possible. What I don’t understand is that the copper doesn’t go anywhere. It was treated with the alpha-acids inside a closed container, which you then drew a sample of for testing. My question is where did the copper go? You should still get the same ppm of copper in the dry hopped sample, correct? Just because it created a complex with the acids, doesn’t mean it went away. It’s still in the keg, but in a complex form. Am I missing something here? Thanks.
BM- I was thinking the same thing. I think the most likely explanation is that the copper and iron precipitated out (perhaps due to a pH change from the dry hopping), otherwise it should have shown up in the analysis of the beer. It’s also possible that the dry hopping complexed the metals and then the hop oils sorbed to the container walls. Both scenarios would remove the metals from the analyzed beer sample.
GWA – I thought about the metals becoming ppt, but this would require that the ions were reduced. I can’t see how the dry hopping would supply the required two electrons to reduce the copper from a +2 state to a 0 state. This would require a current to be run through the keg, and I don’t believe the dry hopping would cause this:)
As for the second option, this would seem more plausible. If the dry hopping oils did create some sort of chelate with the metals, and it did adsorb to the walls, then yes the ppm of copper would have been lower. But again, to go from 5 ppm of copper to 0.23 due to adsorption seems pretty steep. I’m still intrigued as to what exactly is happening here:)
Brett bieren Bij deze bieren worden culturen van brettanomyces, ofwel wilde gisten, toegevoegd voor de hergisting op fles.
I wonder to what extent the experience of copper and the copper levels relate to yeast concentration in samples. As Scott says, the “uptake of copper [by yeast] essentially eliminates it from wort.”