A Look at Studies and Experimental Brew with Tested Results

The past few weeks I have casually been combing through over 20 academic studies on DMS, a topic in brewing that has benefited from a surprisingly vast amount of research and experimentation. Of most interest to me, other than understanding the various aspects of the brewing process that can prevent or lead to DMS in beer, is to determine if DMS is even something brewers need to worry about anymore. If so, how specifically can DMS shape or alter hop aromatics and flavor. The move to higher malt modification has generally created malts that are less susceptible to creating DMS and likely a reason for DMS weighing less on brewers minds. However, as one of the studies detailed below shows, in tested commercial example of ales, DMS was found in amounts above taste thresholds and in comparable amounts as the lagers tested. Could it be that DMS even at low but detectable levels is playing a greater role in shaping the overall flavor profile of beer?
In this post, I first outline the relevant tested information on DMS creation and prevention throughout the brewing process outlined in the academic literature reviewed. I then applied the information to brew an experimental beer doing nearly everything wrong to see if any DMS was actually created. After all, if the beer ends up being fine, despite going against traditional anti-DMS protocol, then this would all be a non-issue anyways. To go further than just taste the poorly brewed beer, I sent three samples to Oregon State University where levels of DMS were tested. The results of the test and tasting notes, including the effect DMS had on hop aroma and flavor are outlined below the research.
What is DMS?
Dimethyl sulfide (DMS) is a sulfur compound that is typically considered an off-flavor in beer at high concentrations and is introduced into beer from the thermal decomposition (wort heating) of S-methylmethionine (SMM) produced in the embryo of barley during germination.1 SMM finds its way into wort because it is readily dissolved from malt at all mashing temperatures. During fermentation, yeast can also reduce another precursor called dimethyl sulfoxide (DMSO), that can turn into DMS in the finished beer. This reaction is by a yeast enzyme called DMSO reductase.2 The extent of this conversion of DMSO is dependent upon the particular yeast strain employed.3DMS can also arise from wort infected with spoilage organisms.4
Aroma/Flavor
DMS is typically described as having an aroma of cooked or creamed corn; however, the literature goes further with descriptors such as sauerkraut-like, black olive, canned corn, cabbage, rotten onions, and blackcurrant buds. DMS is a major contributor to the aroma other beverages and foods including many vegetables like asparagus where DMS is the “dominating character impact compound” and is generally described as having odor sensations of germ-like, mushroom-like, and nutty. 5 White wines, milk, rum, and numerous cooked vegetables are also documented for having a DMS aroma and flavor component.6
Prevalence in Commercial Beers
Most brewers probably don’t think their beer (especially ales) contains detectable levels of DMS, however, an interesting study of thirteen commercially available beers were tested with surprising results. Seven domestic ales, two domestic lagers, two imported lagers, and two non-alcoholic lagers were among the beers tested and found that DMS was at or above flavor thresholds (30-60 µg/L)7 in all but one of the beers, which shows that DMS was contributing to the flavor profile of the beers. Surprisingly, DMS levels in the ales were comparable with the lagers, which are known for producing higher amounts.8 The flavor threshold for DMS is relatively low at 30 µg/L, so it doesn’t take much to contribute to the flavor of a beer.9
Boil
Most of the DMS in beer produced from the SMM precursor is lost (evaporated) during the boiling process. Specifically, it was determined that the half-life of SMM at boiling point is 37 minutes, which means that half of the SMM is evaporated from the boil pot at 37 minutes. A boil of 90 minutes removes approximately three quarters of the DMS and most of the SMM is lost during a 120 minute boil. This half-life of SMM is increased when the temperature is decreased. For example, if wort is heated but not boiled (like a no-boil berliner weisse) to around 190°F, it would have to be held at this temperature for approximately 130 minutes to evaporate half of the SMM precursor.10 The physical location of the brewery can alter the evaporation of SMM because wort boils at lower temperatures at increased elevations, which would result in much less conversion of SMM to DMS in the boil.11
The vigor of a boil also contributes to final DMS levels in beer. One study looked at the power input of a boil and the impact on DMS. The authors found that the when the power input used for boiling increased, the levels of DMS decreased (in a DMS water solution). During a 60 minute boil almost no DMS was detected at 1,500 watts where at 1,000 watts approximately 175 ppb of DMS was detected. At 500 watts, about 300 ppb of DMS was detected. 12 A small electric stove uses about 1,500 watts per hour while it heats on medium or high.13
Whirlpool/Hopstands
During hot stands where the wort remains at hot temperatures after the boil (now typically called hopstands) any remaining SMM in the wort that wasn’t evaporated during the boil will continue to hydrolyse and more free DMS will be formed. One study extended a hot wort stand by one hour and found higher DMS concentrations from an average of around 49 µg/L to 104 µg/L in the extended hot stands.14 This means if a short boil time is combined with an extended hop stand, more DMS in the final beer could be formed. Or in the commercial realm, if a long whirlpool is conducted in a well-insulated vessel or long transfer times occur at hot temperatures, this could lead to a continued breakdown of SMM that survived the boil.
Foam During Boil
One of the most recent studies on DMS looked at the role of beer foam during boiling and the impact of the vaporization of DMS from wort. The authors found that the vaporization of DMS was strongly enhanced in the presence of beer foam versus trials where an antifoam agent was used. Although the direct cause of this was not the point of the study, they speculated that one explanation could be that DMS might actually concentrate in the foam and is then stripped from the wort by the rising vapor of the boil.15
Role of Carbon Dioxide
During fermentation, measured levels of DMS are purged via C02 escaping. Specifically, one study found that there is a significant decrease in DMS levels over the course of the first five days or so of vigorous fermentation, with days 1-2 seeing the most dramatic decreases. However, after day five there was a measured increase in DMS in the fermenting beer, which was produced by the yeast from the DMSO precursor. 16 The Miracle study found an increase in overall sulfur volatiles by the yeast after fermentation was completed with just a short contact time with yeast present, finding that prolonged post-fermentation conditioning in primary can lead to more sulfur-containing volatiles. Specifically, DMS increased to 43.4 μg/L with 30-day residence in a keg vs. 32.6 μg/L with only 14-day residence in the keg.
Why would DMS be created after primary fermentation is complete? Gibson found that when nitrogen is present in the wort in excess a constant and low level of DMSO reductase is detectable. Most typical worts have enough assimilable nitrogen for the yeast, however after primary fermentation and attenuation has been achieved the nitrogen levels in the yeast cells become depleted, which can lead to an increase of DMSO and thus DMS.17 To avoid DMS creation after primary fermentation is over, I’m curious if adding a very small amount of yeast nutrient containing nitrogen around day five would be beneficial.
Open/Closed Fermentation
One study looked at the difference of DMS in beers fermented in an open fermentation system (fermenter with a partially opened top) vs. an enclosed conical vessel. They found that DMS in the open topped fermenter contained the amount of DMS anticipated from the potential of the wort, however, the beer fermented in the conical vessel contained more DMS than could be accounted for from the worts potential. The difference was stark with an ale fermentation resulting in only 9 µg/ in the open fermenter and the conical resulted in 57 µg/ at the time of racking.18 Homebrewers could easily mimic this open fermentation by covering the top of the carboy with sanitized tin foil for the first five days or so of vigorous fermentation then replacing the foil with an airlock for the remainder of time in primary.
Fermentation Temperature
A 1980 Journal of the Institute of Brewing looked at both original gravities of wort and the temperature of fermentation and the effects on DMS in beer. The authors found that worts of high gravities create substantially more DMS than worts of low gravities. Specifically, fermentations of a 1.060 wort had over three times more DMS than a 1.033 wort (adjusted with either glucose or fructose to achieve the higher gravity). With respect to fermentation temperature, it was found that as the temperature increased the level of DMS decreased. The maximum DMS level achieved with a ferment at 46°F was five times greater than a ferment conducted at 77°F.19 This is because DMS production by yeast is greater at lower temperatures.
Gravity of Wort
High gravity worts that are increased in fermentables by the use of sucrose (table sugar) may actually see lower levels of DMS in the finished beer. This is because sucrose can lead to increased DMS volatility. A recent study found that a solution consisting of 10% sucrose (in water) saw a remarkable increase in DMS evaporation.20 However, worts are low in sucrose with typical fermentable sugars profiles of maltose (42-47%), maltotriose (11-13%), hexose (7-9%), and sucrose (2-3%).
Unmalted Cereals
Because barley malt is the main source of SMM, the DMS precursor, the less used in the grist the lower potential for DMS in the finished beer. One way of using less barley is by substituting some of the grist with unmalted cereals, which can also contain DMS precursors, but likely will boil out completely as corn has been shown to do.21 Interestingly, cereals with high gelatinization temperatures, which require boiling prior to usage, may actually have less DMS precursors because this processes breaks down and evaporates DMS, leading to less total DMS precursors in the boil compared to an all malt grist.22
Yeast Storage/Health
The storage of harvested yeast may also play a role in the levels of DMS in beer. A 1985 study examined the DMS levels of beer with yeast stored for 5 days at 34°F under beer (like most homebrewers save yeast) and one stored in water and agitated twice a day and another stored under water receiving constant agitation (via stir bar). Levels of DMS were lower in beers stored unagitated under beer than with agitation. Specifically, the agitated yeast had 25% more DMS in the finished beer which was likely caused by yeast damaged (oxygen toxicity) that may have occurred from stirring resulting in a less vigorous ferment stemming from less healthy yeast pitched.23 This finding is a good reminder to ensure pitching an appropriate amount of healthy yeast, because a healthy vigorous fermentation can aide in controlling DMS Levels.
Hops
DMS can also be introduced into beer through hops. At the end of a typical 60 minute boil all of the sulphur volatiles have evaporated, however during late hoping and whirlpool additions three sulfides peak, including DMS, which is then detectable in the wort. Some of this DMS will be reduced during fermentation via C02, but some could survive and stay in the beer until packaging.24
pH Levels
It was found that worts with a high starting pH can lead to greater amounts of DMS in the final beer. The following chart shows test results of wort fermented with with S. cerevisiae NCYC240 at different starting pH levels.25
| Initial Wort pH | Final Beer pH | Final DMS (µg/L) |
| 4.78 | 3.91 | 65 |
| 4.95 | 3.96 | 76.8 |
| 5.1 | 4 | 84.6 |
| 5.3 | 4.09 | 98.6 |
| 5.46 | 4.12 | 98.8 |
| 5.6 | 4.2 | 94.2 |
| 5.75 | 4.25 | 138 |
Experimental Brew & Results
I decided to brew a NEIPA to test out the research and to see how potential DMS could influence hop aromatics and flavors. Based on the literature, I took the following actions to potentially encourage DMS production:
I aimed for a mash pH over 5.4 which is slightly higher than most typically try to achieve in pale hoppy beers. I tend to like NEIPAs finishing with a slightly higher pH anyways because it seems to smooth out the finish of the beer (less of a bite) by also requiring less direct bittering by hops because beer can actually taste more bitter as the pH increases even when the iso-alpha-content remains the same.26 To help position myself for a higher finishing pH I generally aim for a higher mash pH of around 5.4-5.5, which according to the pH study above, could actually be slightly increase the final DMS content in the beer, which seems to be strain dependent.
At boiling temperatures the half-life of SMM is 37 minutes, so it seemed like a bad idea (or in this case a good idea) to heat the wort up 212℉ and immediately cut the heat. I proceeded to scoop out any foam that was created from the top of the wort as it heated to discourage DMS evaporation (again based on the study above). Because the beer didn’t reach a roiling boil, the foam creation was considerably less than normal, so there wasn’t much to remove. Rather than chilling the batch at this point, I opted to do a 60 minute hopstand, which should give the SMM remaining in the wort from not being boiled out plenty of time to hydrolyse into DMS.
On day five of fermentation, I dry hopped the beer and then capped the fermenter by sealing the 10 gallon keg the wort was in. Learning that after primary fermentation is largely over the nitrogen levels in the yeast cells can become depleted leading an increase in DMSO by the yeast, should this occur, the sealed keg should trap any DMS trying to escape via C02 (as well as potentially retain the dry hop aromatics).
Click to Expand Recipe Details
| Water Prep (100% RO Water) | ||
| Amt | Name | Type |
| 5.75 g | Calcium Chloride (Mash 60.0 mins) | Water Agent |
| 5.55 g | Calcium Chloride (Mash 60.0 mins) | Water Agent |
| 2.30 g | Gypsum (Calcium Sulfate) (Mash 60.0 mins) | Water Agent |
| 2.20 g | Gypsum (Calcium Sulfate) (Mash 60.0 mins) | Water Agent |
| Mash Ingredients | |||
| Amt | Name | Type | % |
| 9 lbs | Organic Brewers Malt (Briess) (1.8 SRM) | Grain | 69.00% |
| 3 lbs 8.0 oz | Malted Spelt (BESTMALZ) (2.4 SRM) | Grain | 26.80% |
| 8.0 oz | Cara-Pils/Dextrine (1.3 SRM) | Grain | 3.80% |
| 0.7 oz | Acid Malt (3.0 SRM) | Grain | 0.30% |
| Mash Steps | |||
| Name | Description | Step Temperature | Step Time |
| Mash In | Add 4.78 gal of water at 161.9 F | 152.0 F | 60 min |
| Steeped Hops | |||
| Amt | Name | Type | IBU |
| 10.00 g | Citra [12.00 %] – Steep/Whirlpool 60.0 min | Hop | 5.4 IBUs |
| 10.00 g | Galaxy [14.00 %] – Steep/Whirlpool 60.0 min | Hop | 6.4 IBUs |
| Fermentation Ingredients | |
| Yeast | Temperature |
| Manchester Ale (Boddingtons) (RVA #132) | 65°F |
| Dry Hop/Bottling Ingredients | |
| Amt | Name |
| 56.00 g | Citra [12.00 %] – Keg Hop |
| 50.00 g | Galaxy [14.00 %] – 5 Days into Fermentation |
| 28.00 g | Amarillo [9.20 %] – Keg Hop |
| 20.00 g | Citra [12.00 %] – 5 Days into Fermentation |
| 15.00 g | Citra [12.00 %] – Pre-Fermentation |
| 10.00 g | Amarillo [9.20 %] – Pre-Fermentation |
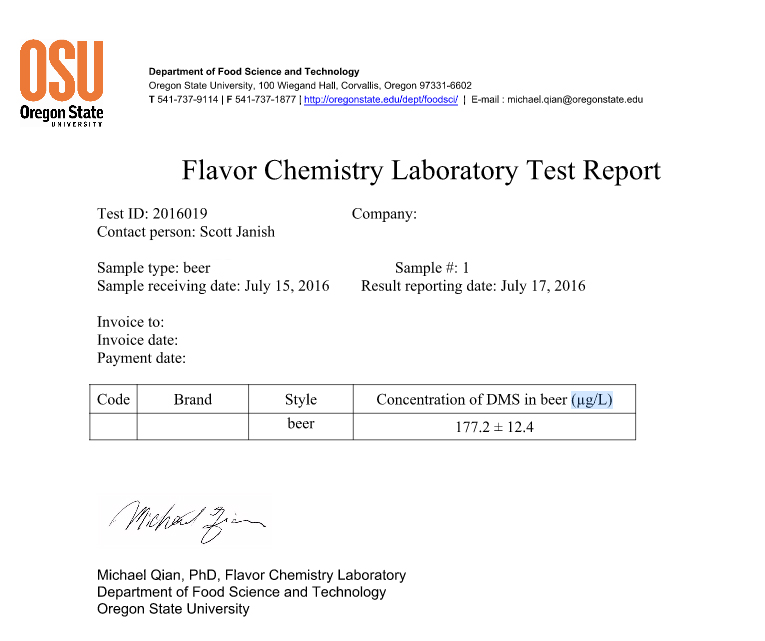
As you can see from the results above, the testing showed a total of 177.2 µg/L of DMS. To put the results into context, ales will generally have less than 30 µg/L, but below 100 µg/L, and anything above 100µg/L is when the flavors associated with DMS start to really come through. When I transferred the beer from the fermenting keg (with C02) to the serving keg with the hops, the aroma coming out of the receiving keg lid immediately reminded me of a hoppy no-boil berliner weisse I made a few years ago. During the first couple weeks in the fermenter the berliner had a very strong musty and briney aroma that made me think I screwed something up. Eventually, with extended time in the fermenter (about 30 days total) and further souring, the aroma did seem to fade quite a bit, but not completely make it’s way out of the beer (I actually enjoyed it, thanks to dry hopping). Now being fairly confident after smelling this experimental beer that what I was likely picking up in the no-boil berliner was DMS, I’m not sure if it cleared up a little from the extended time in the fermenter allowing C02 to push some DMS out or if the flavors and aromas from souring eventually dominated. This experimental beer on the other hand didn’t have additional time in the fermenter (plus it was sealed after day five) and the aroma definitely didn’t fade!
At first pour it’s got a lot of what I would cautiously describe as hop aroma, but with a healthy dose of aromatic hops (Citra, Galaxy, and Amarillo) in two separate large additions, it’s not what you would expect at all. The hop aroma is difficult to describe because it’s being altered so much from the DMS. I’ve settled on calling it an overripe and borderline rotten fruit smell, which sounds about as good as it tastes. This fairly strong “hop” flavor quickly fades though and the briney vegetative aroma comes through even more. Having gotten the results back and knowing that DMS is very prevalent the beer, I wouldn’t say the smell reminds me of creamed corn, maybe this is largely from the dry hopping. To me, the smell is much closer to cabbage and sauerkraut, I can even see how you could describe it as asparagus (based on the research) as opposed to corn.
The overall beer itself is questionably drinkable, it still has a great mouthfeel and all of that, but it’s just so strange. There is no question (and this isn’t really a surprise) that DMS in a high concentration is enough to dominate the hop flavor and aroma. I’m now curious how much DMS might influence hop flavor and aroma in smaller concentrations. For example, if a NEIPA is tested for lets say 50 µg/L, how might even this smaller amount alter the aroma of fresh and fruity hops? I’m particularly interested in this question because I have often thought that as hops fade with time, especially in commercial bottles a few months from packaging, they seem to drift from fresh fruity aromatics to more earthy and vegetative aromas (often I think green onion). I’m not saying for sure this is DMS eventually coming through as the hops fade, but this experiment does seem to convince me that it makes sense to try to keep DMS levels as low as possible in hop forward styles to prevent any sort of aroma competition.
I will likely make a few changes to my process after researching for this article. For example, when it comes to no-boil beers, I think I’m going to start boiling them. I’m already heating the wort for a no-boil beer anyways so why not just let it come to a boil? My memories of that previously un-boiled berliner I talked about really stick out to me as a good example of trying to purge that DMS out of the beer early on.
I’m also going to start experimenting with putting a piece of tinfoil over the small carboy opening on my Big Mouth Bubblers during the aggressive stage of fermentation and replace it with an airlock after around days 3-5. When I ferment in a keg, I might consider leaving the pressure release valve open to mimic an open fermentation to encourage more DMS removal by C02.
The next time I try capping the fermenter when adding dry hops in an attempt to retain hop aromatics, I’m going to think about waiting a few extra days than I typically would to make sure that first big drop in DMS has occurred. I might even try adding a tiny bit of yeast nutrient to boost the nitrogen content again to potentially prevent additional DMS from forming via the yeast.
Footnotes
- Dimethyl Sulfide – Significance, Origins, and Control. (2014). Journal of the American Society of Brewing Chemists ASBC. doi:10.1094/asbcj-2014-0610-01
- S. (2010). Influence of Cardiolipin on Lager Beer Dimethyl Sulfide Levels: A Possible Role Involving Mitochondria. Journal of the American Society of Brewing Chemists ASBC. doi:10.1094/asbcj-2010-0803-01
- Bamforth, C. W., & Anness, B. J. (1981). The Role Of Dimethyl Sulphoxide Reductase In The Formation Of Dimethyl Sulphide During Fermentations. Journal of the Institute of Brewing, 87(1), 30-34. doi:10.1002/j.2050-0416.1981.tb03981.x
- Anderson, R. J. & Howard, G. A., Journal of the Institute of Brewing, 1974, 80, 357.
- Ulrich, D., Hoberg, E., Bittner, T., Engewald, W., & Meilchen, K. (2001). Contribution of volatile compounds to the flavor of cooked asparagus. European Food Research and Technology, 213(3), 200-204. doi:10.1007/s002170100349,
- Badings, H., & Jong, C. D. (n.d.). Headspace Analysis For The Study Of Aroma Compounds In Milk And Dairy Products. Analysis of Volatiles Methods. Applications. Proceedings. International Workshop Würzburg, Federal Republic of Germany, September 28-30, 1983. doi:10.1515/9783110855944.401
- Hysert, D.W., Morrison, N.M. and Jamieson, A.M. J. Am. Soc. Brew. Chem. 37:30 (1979).
- The Measurement of Sulfur-Containing Aroma Compounds in Samples from Production-Scale Brewery Operations. (2005). Journal of the American Society of Brewing Chemists ASBC. doi:10.1094/asbcj-63-0129
- Anderson, R. J., Clapperton, J. F., Crabb, D., & Hudson, J. R. (1975). Dimethyl Sulphide As A Feature Of Lager Flavour. Journal of the Institute of Brewing, 81(3), 208-213. doi:10.1002/j.2050-0416.1975.tb03679.x
- Dickenson, C. J. (1979). The Relationship Of Dimethyl Sulphide Levels In Malt, Wort And Beer. Journal of the Institute of Brewing, 85(4), 235-239. doi:10.1002/j.2050-0416.1979.tb03914.x
- Influence of Wort Processing on Beer Dimethyl Sulfide Levels. (1979). Journal of the American Society of Brewing Chemists ASBCJ, 37. doi:10.1094/asbcj-37-0020
- Dimethyl Sulfide Stripping Behavior During Wort Boiling Using Response Surface Methodology. (2015). Journal of the American Society of Brewing Chemists ASBC. doi:10.1094/asbcj-2015-0103-01
- How many watts does a stove use? (n.d.). Retrieved July 24, 2016, from https://www.reference.com/science/many-watts-stove-use-1717dfe35b2e9bfe
- Dickenson, C. J. (1983). Cambridge Prize Lecture Dimethyl Sulphide-Its Origin And Control In Brewing. Journal of the Institute of Brewing, 89(1), 41-46. doi:10.1002/j.2050-0416.1983.tb04142.x
- Vaporization of DMS Influenced by Foam. (2015). Journal of the American Society of Brewing Chemists ASBC. doi:10.1094/asbcj-2015-0910-01
- The Measurement of Sulfur-Containing Aroma Compounds in Samples from Production-Scale Brewery Operations. (2005). Journal of the American Society of Brewing Chemists ASBC. doi:10.1094/asbcj-63-0129
- Gibson, R. M., Large, P. J., & Bamforth, C. W. (1985). The Influence Of Assimilable Nitrogen Compounds In Wort On The Ability Of Yeast To Reduce Dimethyl Sulphoxide. Journal of the Institute of Brewing, 91(6), 401-405. doi:10.1002/j.2050-0416.1985.tb04364.x
- Booer, C. D., & Wilson, R. J. (1979). Synthesis Of Dimethyl Sulphide During Fermentation By A Route Not Involving The Heat-Labile Dms Precursor Of Malt. Journal of the Institute of Brewing, 85(1), 35-37. doi:10.1002/j.2050-0416.1979.tb06825.x
- Anness, B. J. (1980). The Reduction Of Dimethyl Sulphoxide To Dimethyl Sulphide During Fermentation. Journal of the Institute of Brewing, 86(3), 134-137. doi:10.1002/j.2050-0416.1980.tb03973.x
- Influence of Extract on Volatility of Flavor Components in Wort During Open and Closed Boil. (2016). Technical Quarterly TQ. doi:10.1094/tq-53-1-0212-01
- Russell, I. (2006). Yeast. In F.G. Priest, & G. G. Stewart (Eds.), Handbook of brewing (2nd ed., pp.281-321). New York: Taylor & Francis Group.
- Poreda, A., Czarnik, A., Zdaniewicz, M., Jakubowski, M., & Antkiewicz, P. (2014). Corn grist adjuct – application an influence on the brewing process and beer quality. Journal of the Institute of Brewing, 120, 77-81.
- Yeast Handling Studies. I. Agitation of Stored Pitching Yeast. (1985). Journal of the American Society of Brewing Chemists ASBCJ, 43. doi:10.1094/asbcj-43-0114
- Volatile Sulfur Compounds in Hops and Residual Concentrations in Beer—A Review. (2003). Journal of the American Society of Brewing Chemists ASBC. doi:10.1094/asbcj-61-0109
- Anness, B. J., & Bamforth, C. W. (1982). Dimethyl Sulphide-A Review. Journal of the Institute of Brewing, 88(4), 244-252. doi:10.1002/j.2050-0416.1982.tb04101.x
- Meilgaard, M., and Trolle, B. The utilization of hops in the brewhouse. Proc. 6th Congr. Eur. Brew. Conve., Copenhagane. 1957

-610x915.jpg)
Great article. Thanks!
Great article as always, but when people will understand that American fahrenheit is the exception and not the rule. It’s so tiring to have to convert F to C all the time!
Great write up! We’ve done some review of DMS studies on the MTF wiki in an attempt to explain why anecdotally many brewers seem not to get DMS in no-boil beers. Calculations were based on new studies on the thermodynamics of DMS volilitization by Scheuren et al. Using their proposed calculations, we predict very low amounts of DMS in various approaches to no-boil (a member of the group who is a physics professor performed the calculations for us and generated the graphs). Two variables seem to have a great effect: the fact that SMM converts very slowly to DMS under 95°C, and that DMS is also very volatile at temperatures between 50-100°C. I’d be curious of what your thoughts are on this: http://www.milkthefunk.com/wiki/Dimethyl_Sulfide#DMS_Prediction_Models
This is incredibly well researched and informative, I love the work you guys are doing! I’m having a hard time understanding how with such high starting SMM figures of a no-boil (920 µg/L with the 35 min. calculation) that the actual DMS levels are so low. I must have something to do with The decomposition caused by heat above ~80°C (I saw on your webpage) that is factoring into the calculation from “Principles of Brewing Science: A Study of Serious Brewing Issues”, by George Fix (I need to read these pages I think). I wasn’t able to read anything showing ~80°C figure, do you have any references? I’m not disputing this at all, just trying to understand it. I don’t want to mislead anybody and need to update what I have or reference your calculations for no-boil beers. Has anybody brewed a beer and tested the DMS levels with these calculations?
Thanks for the kind words! Yes, this comes down to the half-life of SMM at different temperatures. According to “Dimethyl Sulfide – Significance, Origins, and Control. Charles W. Bamforth. 2014.”, the half-life of SMM doubles for every 6°C drop in temperature. At 100°C and 5.2 pH the half-life is ~35 minutes, but at 82°C the half-life is ~300 minutes (see the “SMM Precursor” section on that wiki page). So, if you bring a wort to 82°C for pasteurization purposes, and hold it there for 15 minutes and then cool it, very little SMM is decomposed into DMS during that time and temperature. This results in a wort with high SMM, but insignificant DMS. I have yet to find a reference on the flavor effects of SMM (if you know of any, please let me know; I believe there may be some German texts that address it).
We have not yet done any real life tests to see if the implementation of the Scheuren formulas, and a replacement of George Fix’s calculation that seems to unfairly represent the SMM half-life at different temperatures. Fix’s calculation represents DMS in real world situations very well when compared to normal 60 minute boiling techniques, but in the case of what we are doing with no-boils, it just isn’t a fair calculation. Hammond’s computer program substituted this and used the actual SMM half-life values for whatever the temperature is at every 0.6 second interval.
The Scheuren calculations, which calculate the volatility of DMS at below 100°C have been compared with real life tests and deemed accurate, according to his studies.
So, if you bring a wort to 82°C for pasteurization purposes, and hold it there for 15 minutes and then cool it, very little SMM is decomposed into DMS during that time and temperature.
=> I heard SMM coverts to DMS with little heat, even in mashing temp.
So how could we be sure that those high amount of SMM not chaning to DMS while holding 15mins at 82’c? It’s not relevent to the half life of SMM, is it?
Do you have any reference about SMM to DMS decomposing rate in diffrent temp?
Thanks for putting this together! It’s an interesting read. I’ve had 2 batches with noticeable dms and they seem to match this info. One was a split batch of pale ale & ipa where the wort for the pale ale was pulled off after only 45 min of boil then I added some extract and hops to boost the remaining 5 gal to be an ipa and boiled 15 more min. i also dry hopped The pale at day 5 and capped it. The pale had a cabbage aroma & flavor that made the hops taste alittle onion-garlicy while the ipa half did not taste off at all. The other batch was a split batch of cream ale made with a friend of mines test batch of craft malt. I added some table sugar to half of it to dry it out more and while both halves had a corn flavor it was definitely less in the half with sugar added.
That sounds about how the batch I made tasted with very high levels of DMS. Capping at day 5 probably had something to do with an increase in DMS being trapped with low nitrogen content left in the wort for the yeast, which can then lead to more DMS. I think I might wait until day 7 or so to cap and try adding a tiny bit of nutrient, cant hurt right!
scott – nice detailed write-up. did you cover the kettle when you did the hopstand? i have heard anecdotally that covering the fermenter during a stand would not allow the smm/dms to volatize and it will catch on the kettle lid and drip back down.
but it seems like you are inviting lord knows what into your wort if you keep it uncovered for a 60 min hopstand.
dan – That is true. Cooling from 100°C until pitching temperature in an open system is a good way to get a contamination. The graphs were designed this way to demonstrate how DMS is volatilized in an open system where temperatures fall below 100°C using proposed formulas from Scheuren et al. You can also use the tables to get a visual representation of how much DMS would be retained by starting off with an open cooling system, and then covering it at any point in the process (follow the curve on the DMS line as the wort is cooled). In our practical recommendations for preventing DMS formation (if it is a problem), we advise trying to cool the wort openly until around 60-70°C, which is still hot enough to keep the wort pasteurized, and then closing the system to prevent contamination before the temperature drops below 60°C. See the “Avoiding DMS” section on the MTF wiki .
Sorry, I thought that question was directed at me. I believe maybe you were directed it to Scott.
Any thoughts on surface area to volume ratio? This is a large difference between homebrew and pro kettles. Or perhaps kettle depth? Deeper kettles are more difficult to ‘circulate’. I am very curious your DMS measurement on a ‘normally’ brewed beer. Anecdotally DMS is not an issue for homebrewers (who aren’t trying to maximize their DMS ;)).
I didn’t see any direct look in the studies on surface area to volume ratio, but you would think a larger surface would lead to more evaporation of DMS. I did read a study that the MTF page cited saying, “the total DMS present in the wort would eventually be evaporated off regardless of what the top surface area of the kettle.” A quick re-read of the text and I’m not seeing this conclusion, but I trust that it must be in there.
This was an interpretation of the follow paragraph in the Scheuren review: “A very common statement, that the surface would influence evaporation efficiency by increasing the separation factor, can also be refuted based on the shown considerations. None of the mentioned equations contain surface at any state. This clearly illustrates that an impact of an amount, such as to change the conditions of phase equilibrium, is not possible by an increase of the surface. It is just the mass transfer per time that is affected, not the mass transfer itself.”
I could be misinterpreting this paragraph (Dr. Hammond did not disagree with me when I brought it up with him), but I interpreted it to mean that X amount of DMS will always evaporate off with Y amount of evaporation. However, with a larger top surface area evaporation is faster, and in a practical sense DMS evaporation is faster too. So, surface area doesn’t effect the volatility of DMS, it just effects how fast the volatilization occurs over time. I would be interested if you disagree, I can always take this back to Dr. Hammond for some input.
Cheers,
Dan
I’m sorry Dan, I was looking at the wrong Scheuren paper (Influence of Extract on Volatility of Flavor Components in Wort During Open and Closed Boil) also from 2016. No wonder I didn’t see what you have posted!
This is very interesting and I would agree with how you have interpreted the paragraph you shared. It too sounds to me like a time issue, which I would think could be relevant information if you choose to do shorter boils, but to what extent based off surface areas I’m not sure. I’m glad you could clear this up for me. Thanks for sharing and clarifying!
I am thinking about using metabisulfite during the whole process of making beer. Originally i added only a small amount (0.1g/10l) to the brewing water to remove chlorine but now it seems like it can even help my mash. Do you think it would be useful to add it to the primary fermenter during dryhopping and at bottling time? (to help fighting oxidation during bottlecarbing)
(sorry for the offtopicish question, a simple yes/no answer can be fine)
Whoops I just realized that my post was completely off-topic, there was a recent article I was thinking about during writing the post.
http://immaculatebrewery.com/oxygen-and-mashing
Hi! Very nice read! Thank you!
Any idea if the same holds when brewing with extract as well?
I did a little experiment. I used the same recipe (hops, dry malt extract, yeast) in two batches. One I boiled for 60+ minutes, the other one for 20ish minutes. The recipe is my session beer, with 5% ABV, fermented at around 60 F.
After bottling, the 60 min brew clarified much quicker than the 20 min.
However, after 3 weeks, there was virtually no difference between the two beers. Not in taste, clarity, head retention, etc.
I know it’s just one data point, but i’m going to try more of the 15-25 min boils.
Is this surprising? Are my taste buds off?
It’s my understanding that extracts are boiled for a bit and should have some DMS already driven off. Because of this, I would think extract wouldn’t require much of a boil time to drive off DMS.
I work in a regional brewery of about 170,000 hl per annum. We collect the fermenting gas (during active fermentation), clean it and liquefy it, then reintroduce it in bright beer tanks for our desired CO2 level. Can you comment on DMS from collected fermenting gas if the scrubbing process is not adequate.
Thank you
That sounds like a great setup! I can’t say I’ve read anything specific to any DMS that might be collected as part of the fermenting gas recapture or if it would even stick around after liquefy it, but I’m just as curious as you now!
http://www.agraria.com.br/extranet/arquivos/agromalte_arquivo/controle_de_dms_no_co2_da_cervejaria_-_ing.pdf
this ought to answer those questions.
I know that I’m late to the party but you mention the evaporation of SMM in your paper. The Scheuren et al. article claims that SMM and DMSO have a very high boiling point and thus cannot be removed during the wort boil. I found the boiling point of DMSO (189C) but I can’t find evidence whether SMM can be evaporated or not. Can you help?
So you keep wort temp under 80C but above 37C and assuming that one can get evaporation, all of the DMS is gone and no additional SMM is degraded. Any flavor contribution of SMM aside, am I correct to be nervous about leaving so much non-degraded SMM in the beer just waiting on something to degrade it. SMM does not seem like the most stable compound.
It was a very interesting article and I will adjust my approach to DMS. However, I have been a big fan of pressurized fermentation – usually around 10-15 PSI – I have not noticed any off flavors but my Hops are not exactly screaming out flavor either and I dry hop around 4-6 g/L. I wonder if the DMS could be dampening the HOP aromas . . . . or maybe something else is going on with the pressurized fermentation? I would be very thankful for any remarks you may have on this subject
“Pressurized Fermentation and DMS”