A Look at pH in Hoppy Beers
Heavy dry hopping will increase the final pH of beer, but does it matter?

A Look at pH in Hoppy Beers
H ow important is the final pH of beer? What factors can play a role in altering the final pH of beer? What are the pH ranges that people generally prefer in beer? Other than taste, what other impacts does high beer pH have in hoppy beers? These are a few of the questions I try to examine in this post by looking at recent research. In addition, I created a draft calculator that can estimate the impact dry hopping will have on your finished beer pH.
During the past couple of years there has been an increasing amount of research done focused on how the increase in dry hopping rates is impacting hoppy hazy beer. Factors like beer bitterness and pH is among some of the focus of the studies. I’ve received an increasing number of questions surrounding some of these issues, like should we be lowering the final pH in some of these hop-forward beers? Should we even care about the higher pH in the hazy style? I didn’t have great answers so I turned to the research to see what I could find out. Unfortunately, I still haven’t come to any conclusions as to the best way to approach pH in hoppy beers (I think that’s kind of the point of digging further into our beers), but hopefully this post can help inspire some new experiments and start discussions among brewers about how they are thinking about the higher beer pH in the hazy style.
How Dry Hopping Rates Increase Beer pH
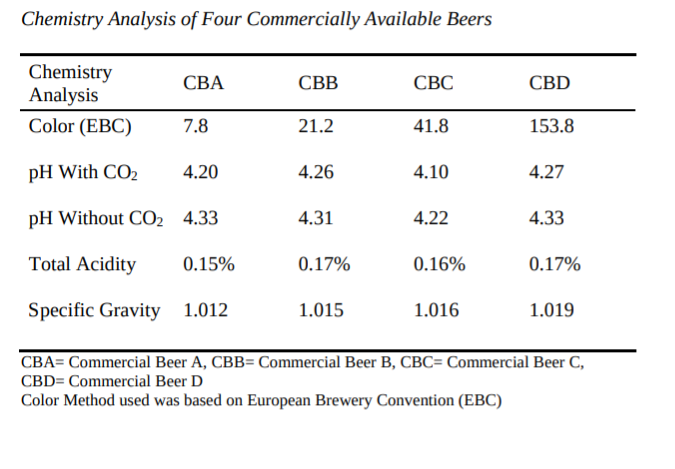
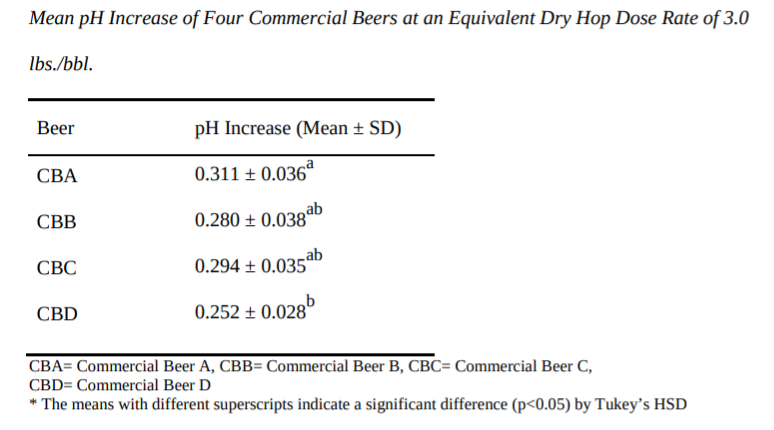
Starting with a 2014 paper by Matthew Schmick, titled “Dry Hopping and its Effect on Beer pH” which was one of the earlier papers I found on the topic Schmick found that that dry hopping at two different rates (0.5 pounds/bbl and 3 pounds/bbl) resulted in different increases in pH. Although the exact increase among the six different hop varieties used was slightly different, there was mean pH increase of between pH 0.040 ± 0.003 and 0.056 ± 0.006 at 0.5 lb./bbl. of dry hops. When jumping to 3 pounds per barrel, there was a mean pH increase of pH 0.233 ± 0.022 and 0.332 ± 0.031. So, as the dry hop increases in pounds/barrel the rise in pH is slightly reduced.1 The findings would align with what John Paul Maye found in more recent research, which saw a lower final beer pH in west coast IPAs than from NEIPA beers that were dry hopped at higher rates.
Also interesting in Schmick’s paper is the impact specific gravity had on the rise in pH from dry hopping. As you can see from the two charts below (citation above), the commercial sample with the highest final gravity (1.019) resulted in less of a rise in pH from dry hopping (negative correction). The results could also help to explain why NEIPAs may see slightly less of a pH rise as many finish with a rather high gravity (at Sapwood we often finish around 1.020+).
Three additional papers have directly looked at the role of dry hopping and its implications on the pH of beer. A 2019 BrewingScience paper found that each dry hop dosage would result in a consistent increase in pH of 0.03 for every 100 g/hl of hops used.2In 2016, Dr. John Paul Maye led a study that determined dry hopping at a rate of 100 g/hl would raise the pH of beer 0.036.3
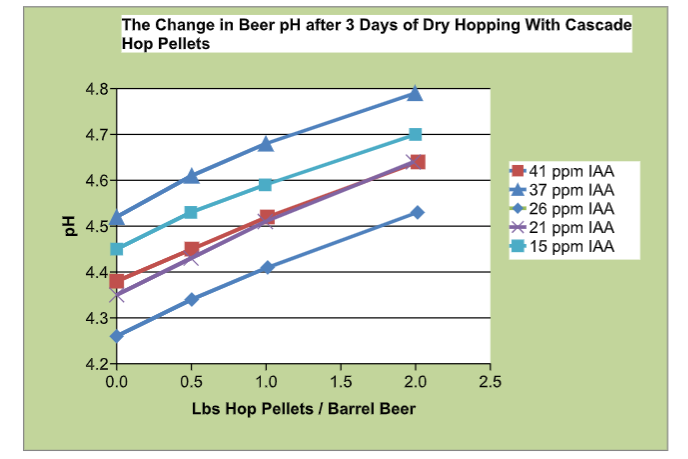
Maye’s results also align with a 2018 paper that also found a linear increase of 0.036 pH increase per 100 grams of dry hops used.4 Below is a chart from Maye’s work when testing beer pH after dry hopping with Cascade up to 2 pounds per barrel (cited above).
I reached out to Dr. John Paul Maye to ask further questions regarding the increase in pH coming from dry hops. Maye explained to me that his team did a lot of work trying to isolate the compound(s) in hops responsible for the increase in pH, but they haven’t yet determined the exact cause. They did find that that the rise in pH is coming from something in the leaf material of the hop and not within the lupulin gland and is extremely water-soluble. Maye came to this determination because when they dry hopped samples with CO2 extracted spent hop material it would cause an increase in beer pH at the same rate as unextracted hops. Potentially, when dry hopping with Cryo hops which have had most of the vegetal material removed, you could experience a smaller increase in pH than what you would expect from T-90 pellets.
As mentioned previously, Maye’s research found that even though hazy IPAs (which have much higher dry hopping rates) had slightly lower pHs on average than west coast IPAs (with lower dry hopping rates). The reason could be that active fermentation dry hopping, sometimes done in hazy IPAs, is giving the active yeast a chance to absorb or metabolize the pH increasing compound(s) coming from the plant material of the hops. I also wonder if the higher gravities of NEIPAs as well as dry hopping rates above the 2 pounds per barrel (as studied in the chart above) are playing a role to help explain the west coast IPA to NEIPA pH differences.
Dry Hopping pH Calculator
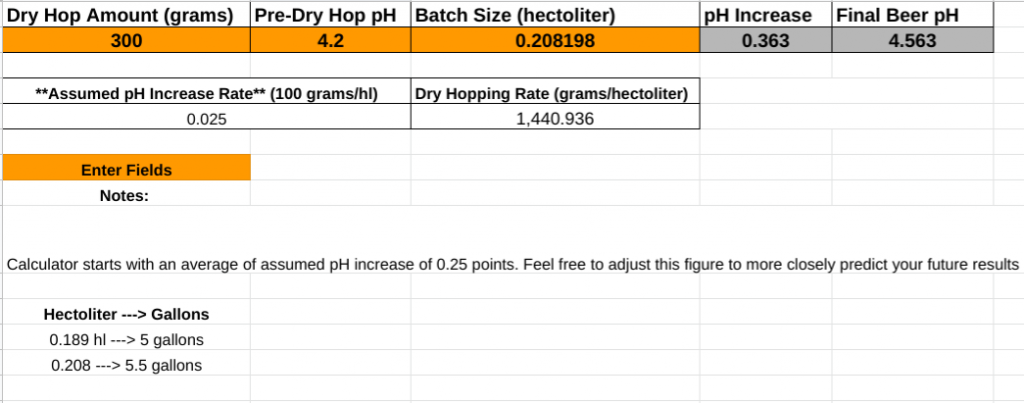
Using the data from the above studies, I put together a draft calculator which can help estimate your final beer pH based on your dry hopping rates. Specifically, the calculator assumes that dry hopping at a rate of 100 g/hl (0.26 pounds per barrel) will increase beer pH by 0.025 units. Although some of the research described above found the increase to be closer to 0.036 units, I’ve found this is too high based on my experience and playing with the calculator. The figure I use is closer to what was found in the higher gravity sample from the 2014 Schmick paper. To use the calculator, enter your dry hop amount in grams, batch size in hectoliters, and the pH of the beer pre-dry hopping (post fermentation), and it will calculate an estimated pH increase. Feel free to play with the assumed pH increase rate to dial this into your actual results to more accurately predict future beers.
Does it Matter?
What matters most is how the beer tastes, right? What happens when the same dry hopped beer is left at the higher pH or lowered with a form of acid and put to a panel of tasters. Maye’s team did this and found that the tasting panel found the higher pH beers scored higher for bitterness marks as well as for overall enjoyment. Specifically, they lowered the pH of the dry hopped beers from a pH of 4.9 to 4.5 using sulfuric acid and the lower pH beers didn’t taste as good as the higher pH beers. The chosen method of acidification (sulfuric acid) could be the reason for the tasting preferences (I chose lactic acid in my tests).
There is not a lot of research that I could identify on the impact of manually lowering beer pH in heavily hopped beers pre-packaging and how this might translate to positive or negative sensory scores. Because of this, I had few of us at Sapwood taste two of our hoppy beers dosed with lactic acid after packaging.
First, we took our hazy 8.6% DIPA (Cryovolcano) that was dry hopped at 4.4 pounds per barrel (~16g/L) and took a final beer pH reading, which was 4.56. Using an eyedropper, I dropped in lactic acid until reaching a reading of 4.2. We did one blind triangle test and could identify the dosed beer out of the lineup. The consensus was the beer with the lower pH was slightly softer on the palate with more of a “hop compoundy” flavor. In contrast, the higher non-dosed pH beer finished with a little more of a green polyphenol-like bite with a lingering herbal quality. For me, the lower pH beer invited another drink sooner, and I preferred it out of the two, but I had a hard time deciding.
Moving to a 5.6% hop-forward beer dry hopped at a rate of 2.2 pounds per barrel (~8g/L), I again measured the final pH of one of our beers (Light and Beauty) reading 4.52. Using lactic acid, I dropped in enough to get the same 4.2 reading. Again, it was fairly obvious to pick out the lower pH beer, even more so in this lower ABV beer. The lower pH beer had less of an aromatic punch (although some of the aromatics could have been driven out while mixing in the lactic acid). Much like with Cryovolcano, the lower pH beer finished with less harshess/jaggedness. However, I think this time at the detriment of the beer. Here the higher pH beer was perceived to have more body and complexity to me, and the lower pH sample tasted a little flatter in flavor. In this case, I favored the higher pH beer.
Although this is just a couple of samples and only using lactic acid (tartaric acid could be another option to experiment with), it was interesting to taste how adjusting the final pH could impact the beer. Perhaps lowering the pH to just 4.4 in the lower ABV (or even higher) beer would have been a better sweet spot. Practically speaking, I also wonder if reducing the pH pre-packaging in a big, heavily dry hopped DIPA might allow for slightly higher dry hopping rates because the acid seemed to help lower the green-polyphenol finish a bit.
Shelf Stability and High Beer pH
There is some concern with beers that finished higher in pH and its microbiological stability. Beer is generally not the greatest environment for most microorganisms to take hold as beer is usually has acid with pH’s ranging from pH 3.8 to 4.7, lower than most bacteria can tolerate for growth.5Most heavily hopped beers should have less bacteria-related problems (mainly from gram-positive bacteria or lactic acid bacteria). The reason is that the minimum concentration of hop acids needed to inhibit most gram-positive bacteria is coming from the high hopping rates. Examples of gram-negative bacteria of the genera Pectinatus and Megasphaera that could potentially still emerge, but lower pH and increased alcohol helps to inhibit them. So, the enormous amount of hops used in higher finishing pH NEIPAs should help to inhibit lactic acid bacteria anyways.
Head Retention and Beer pH
A paper that looked at how dry hopping impacts head retention and found that as the pH increased in beer the head retention decreased.6 In the paper, Cascade hops were used for dry hopping at dosage rates of 0.5, 1, 1.5, 2, 2.5 lbs./bbl and measured for foam stability. As the dosage of Cascade increased, foam stability decreased as the pH increased. In addition, the longer Cascade hops sat in the beer during dry hopping, the more the foam stability was reduced. This decrease in stability was slight after two days of dry hopping, then accelerated on day three, and continued to slowly decrease until day eight. Ultimately, long term dry hopping can have a negative impact on head retention. Another point in favor of shorter short dry hop times.
Using the NIBEM test for beer foam, a 2019 BrewingSciencepaper (also referenced above for pH increases from dry hopping) found a reduction in foam stability at higher dry hopping rates with T-90 pellets. Specifically, the authors found a definite drop in foam stability with an average of 91 seconds less. The result is likely attributed to foam positive proteins interacting with the hop derived polyphenols and the vegetative matter in the hops pulling out foam positive iso-alpha-acids losses during the dry hop. Also, dry hops contain foam negative waxes, hop oils, and lipids that can impact head retention.
Beer pH and Ester Production
What about beer pH and its impact on ester production? Although less of a concern when making clean (non-soured) hoppy beers, it’s interesting to learn how pH can impact fermentation. The Journal of Bioscience and Bioengineering tested three different beers fermented each with a different starting pH (3.0, 5.0, and 7.0). As the starting pH increased, so did the esters formed during fermentation.7 The beer fermented at a pH of 7.0 resulted in the highest ester concentration with a 13% increase in total acetate esters and a 7% increase in total ethyl ester production compared to the control (5.0 pH beer). On the other hand, the beer fermented at a low 3.0 pH resulted in an 18% decrease in total ester concentration. Not entirely useful information when making NEIPAs, but still interesting!
Beer pH and Haze Stability
It appears that both the pH of wort (no alcohol present) and beer can impact the haze levels in your beer. A 2003 Journal of the American Society of Brewing Chemists paper determined that the pH of beer can significantly impact the protein-polyphenol interactions leading to haze. The authors found that the highest haze intensity increased over seven-fold as the pH increased.8 Specifically, with no alcohol present, the maximum haze was found in a pH range of 5.0-5.5. With alcohol present, the pH range where the most turbidity was found decreased. With an ABV of 6%, the pH range with the most haze potential was 4.5-5.0, and with an ABV of 12%, the pH with the most haze potential was 4.5.
To me, this suggests that day one dry hopping could potentially lead to more haze in part because post-boil beer pH (generally around 5.0-5.2) falls nicely into the range the paper above determined has the highest haze potential from protein-polyphenol interactions. It’s interesting then to experiment with small day-one dry hop charges to encourage haze stability (if this is what you are after). This small initial dry hop dose might not contribute much to the aroma of the beer as I would expect most of the compounds (especially the less-polar hydrocarbons) would be removed during fermentation. Still, a small amount of the fruiter and more polar monoterpene alcohols may remain.
I’m curious if any of you are tracking pH throughout the processes? Are you making any adjustments to the pH pre-packaging? If so, how and why?!
Key Findings
- Dry hopping will increase the pH of your beer. Research has found the increase to range from 0.025–>0.036 pH units. Huge dry hopping rates may lower the pH increase as well as higher finishing gravities.
- The rise in pH coming from dry hopping is likely due to something in the vegetal material in the hops (not the lupulin gland) which suggest that the pH increase from Cryo dry hopping may be less than T-90 pellets because most of the vegetal material is removed.
- In relaxed sensory trials, I preferred the lower pH beer in a heavy dry hopped DIPA, but not in the lower ABV session hop-forward beer. The drop in pH seems to lower the green-polyphenol bitterness (which somewhat agrees with research that higher pH can lead to more bitterness). However, in the lower ABV beer the drop in pH took away from the complexity and negatively impacted the beer (in my opinion).
- As the pH in beers increases, the head retention can decrease.
- Protein-polyphenol interactions that lead to beer haze is impacted by pH. In a moderate 6% beer, the peak range for interaction is between 4.5-5.0 pH).
Footnotes
- Schmick, M. J. (2014). Dry Hopping and its Effect on Beer pH. University of Wisconsin-Stout, Graduate Paper School.
- Cocuzza, S. (2019). The impact of dry hopping on selected physical and chemical attributes of beer. BrewingScience, 72, 118–124.
- Maye, J.P., and Smith R. and Leker J.S.: Humulinone Formation in Hops and Hop Pellets and Its Implications for Dry Hopped Beers, Master Brewers Association of the Americas, 53 (2016) no. 1, pp. 23-27.
- Lafontaine, S. and Shellhammer T.: Impact of static dry-hopping rate on the sensory and analytical profiles of beer, Journal of the Institute of Brewing, 124 (2018), no. 4, pp. 434-442
- Kanta Sakamotoa, Wil N. Konings, Kanta Sakamotoa, Wil N. Konings, Beer spoilage bacteria and hop resistance, International Journal of Food Microbiology. (n.d.), 2002.
- Wilson, R., Schwarz, H., & Maye, J. (n.d.). A Natural Foam Enhancer From Hops. Speech presented at World Brewing Congress 2012, Portland, OR.
- Hiralal, L., Olaniran, A. O., & Pillay, B. (2014). Aroma-active ester profile of ale beer produced under different fermentation and nutritional conditions. Journal of Bioscience and Bioengineering, 117(1), 57-64. doi:10.1016/j.jbiosc.2013.06.002
- Karl J. Siebert & P.Y. Lynn (2003) Effects of Alcohol and pH on Protein-Polyphenol Haze Intensity and Particle Size, Journal of the American Society of Brewing Chemists, 61:2, 88-98, DOI: 10.1094/ASBCJ-61-0088

-610x915.jpg)
I find that lower ph in neipas does the trick in simulating fruit juice. Thats why I prefer kettle soured neipa, with lacto also enhancing citrus flavors. I also find sour beers much less astrigent than normal neipas. So either I kettle sour before whirlpooling, or add some citric acid before whirlpooling and dry hopping.
Same thing here. We lower the pH in the kettle on our NEIPAs. From a visual evaluation, they seem to be more stable hazevise by doing so.
Scott, love what you do and thank you for so generously sharing all of this information. My question is this: If I wish to accurately measure pH of a finished beer (for possible adjustment), do I need to degas the sample for measurement? Does carbonation (CO2 in solution) alter the pH of the beer significantly?
I would think for sensory that you want to measure pH exactly as it is hitting the taster’s palate (i.e., at serving carbonation level). Just curious if you feel carbonation is a significant factor and how you handle that in your experiments. Thanks!
How can I know how much lactic acid I am going to use to lower the pH to a specific pH?
Hey! I take a small measured sample and dose that to get to the pH I’m after, then I scale up the dosage rate for the amount of beer I’m trying to adjust.
Late to the party…but..
I started dosing my NEIPAs at the end around the same time this article was published (when dry hopping) with lactic or phosphoric acid to combat dry hop pH increase. I found I needed to add around 0.27ml per Litre of 88% phosphoric acid to bring a beer down 0.2-0.3 points so it sits somewhere in mid 4.30s where it finished before adding my usually dose of 16g/L T90 pellets (the vagueness is because it does vary abit depending on FG, minerals etc….my beers are round 8% abv, a lower ABV beer may require less acid but may be impacted more by dry hop pH increase)
In pre-dose tests I found exactly what you described in this article:
Beers with acid adjustment were just as bitter on the tongue at first but the bitterness didn’t linger and the grassy finish was reduced giving overall impression of less bitterness. I found the hop taste was reduced and the malt/hop taste complexity a little down. I also found slightly less aroma. I did add to a control sample water and stir so I can confirm it’s not the stirring thats the cause. Very strange.
I did find when adding more than 0.3ml per L of 88% phosphoric acid the beer started to taste like it had been dosed with acid, so I wouldn’t go higher. Lactic acid worked well, but found the phosphoric acid was marginally cleaner and you have to add less as its a stronger acid.
My best NEIPA to date was dosed with acid but I’m going to switch back on my next brew to not adding acid and reduce boil hops to compensate for bitterness as I think the complexity and aroma suffered slightly.
Very interesting article, thanks! You ask what people are doing re pH pre-packaging. I recently fermented a Brut IPA with Lallemand Voss Kveik, which is known to ferment out a little more sour, and ended up at 3.15pH post fermentation, which was too sour. After dry-hopping at 11g/l raised the pH to 3.68, which was still too sour for our taste. We taste-tested 100ml samples adjusted with Sodium Carbonate („washing soda“) to different pH, and found the sample with 0.5g/l Sodium Carbonate tasted best and had a pH of 4.2. I added the corresponding amount along with the priming solution pre-packaging and were pleased with the result.
Hi again. This topic came up again in the forum and a brewing chemist acquaintance of mine sent me this article which found (as I did empirically) that *raising* pH lowered the perception of astringency.
https://www.sciencedirect.com/science/article/abs/pii/S0950329305000807
I had a NEIPA in the FV that had a (too) lowish pH of 3.9 and after DH‘ing with 12g/l @ 20° (but before cold crashing) also had too much polyphenolic astringency.
However, I found out that after correcting the pH to 4.2 with 0.2g/l NaHCO3 that the perceived polyphenolic astringency was much less.